
A research team led by Prof. ZHANG Haimin from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences has conducted a systematic study on the regulation mechanism of heterostructure bimetallic phosphide electrocatalysts to improve the performance of electrochemical nitrate reduction reaction.
"The catalyst shows different ammonia synthesis performance in different reactors," said Dr. JIN Meng, "so we tried three different electrolyzers to improve the performance of the electrocatalysts."
The configuration of the electrolyzer will greatly affect the local reaction environment near the electrode, and then affect the catalytic performance, the researchers chose three different electrolyzers to study the regulate mechanism of the electrocatalysts performance.
Results were published in Nano Research.
Nitrate anion (NO3-) is significant pollutant in industrial wastewater and agricultural production. Electrocatalytic nitrate reduction (NO3RR) is an effective way to solve environmental problems and produce green ammonia (NH3). However, the NO3RR process is complex, involving multiple electron and proton transfer, and suffers from low Faraday efficiency due to competition from hydrogen evolution reactions. An electrochemical NO3RR reactor is also crucial for achieving high-efficiency conversion of NO3- to NH3.
In this study, the researchers synthesized a series of bimetallic copper-nickel phosphide electrocatalysts on commercial carbon paper (CP) by a facile vapor-phase hydrothermal method. The electrocatalytic performance of these catalysts for NO3RR was first evaluated in an H-type electrolytic cell. The results showed that the Cu3P-Ni2P/CP-x could form rich heterointerfaces, which enhanced the electron transfer and improved the NO3RR efficiency.
To further understand the difference in NO3RR kinetics of electrocatalysts, the researchers used a rotating disk electrode setup to test the corresponding catalytic kinetic parameters.
In addition, they assembled the catalyst into the membrane-electrode-assembly (MEA) electrolyzer, which demonstrated the highly efficient activity and durability for NO3RR at industrial current densities. In-situ spectroscopy characterization, combined with theoretical calculations, revealed that the presence of heterointerfaces effectively regulated the reactant adsorption, and the reaction mechanism followed a sequential hydrodeoxygenation pathway.
These results contribute to a better understanding of the electrocatalytic NO3RR process and pave the way for the development of efficient and durable catalysts for sustainable ammonia synthesis.
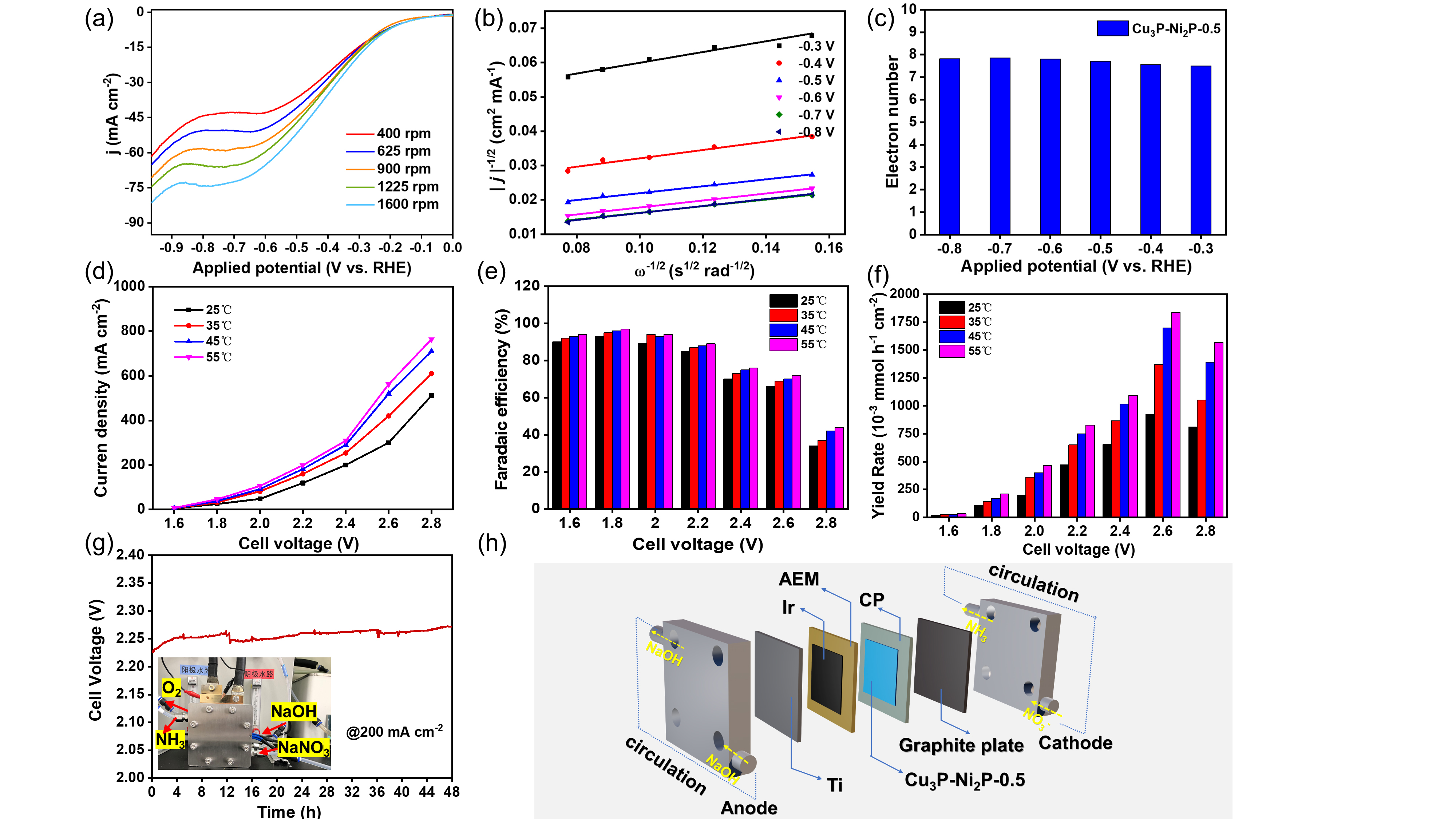
The electrocatalytic NO3RR kinetic test and its scale-up for practical applications. (Image by JIN Meng)
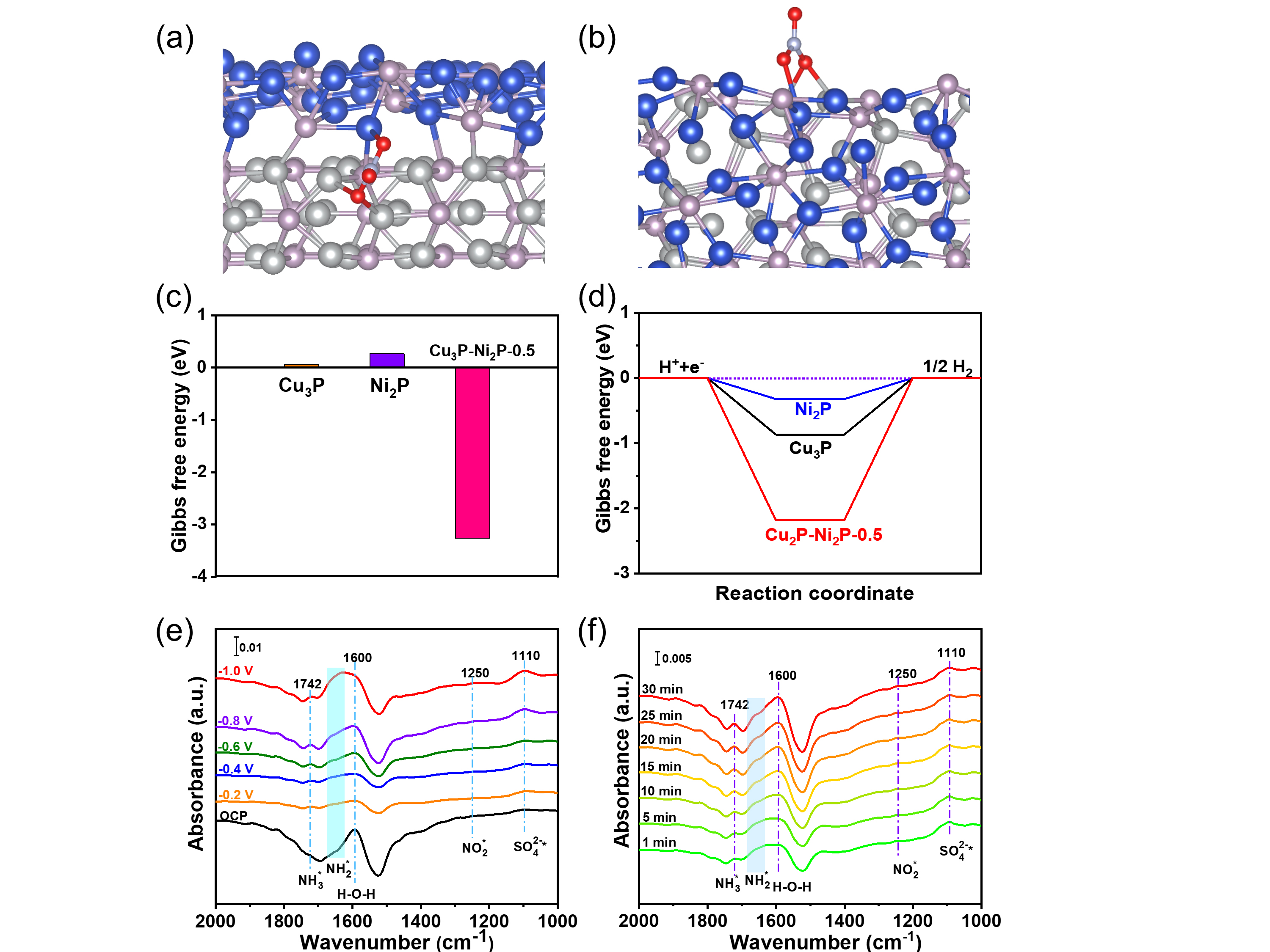
Theoretical calculations and In-situ spectroscopy characterization toward NO3RR. (Image by JIN Meng)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

